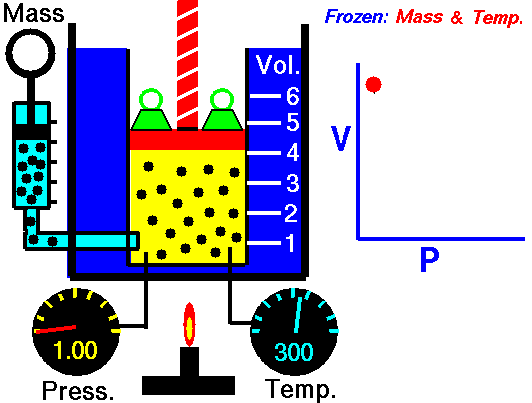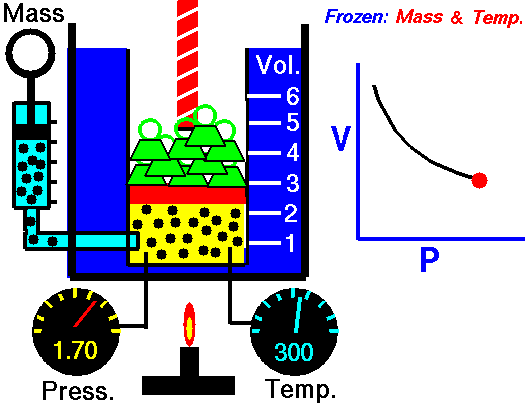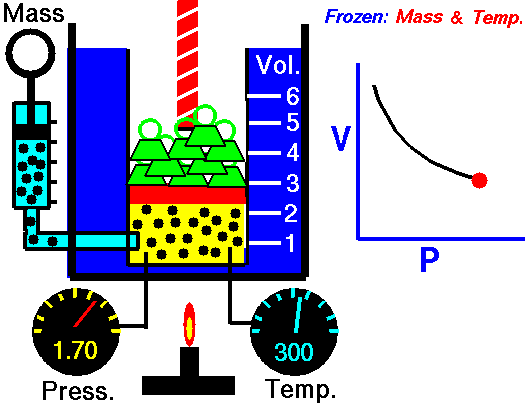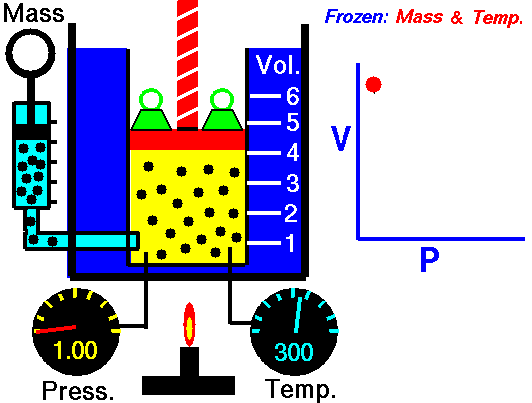
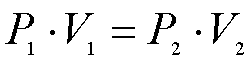The volume of a gas is inversely proportional to the pressure at a constant temperature
For example, if you double the pressure, the volume will be cut in half. If you increase the pressure ten times, the volume will decrease 10 times.
This means the following conditions are required:
- The amount of gas is definite.
- The temperature (in Kelvin) is the same.
- The gas is in a closed system.
According to Boyle's Law
- As the volume of the gas increases, the pressure of the gas decreases.
- As the pressure of the gas increases, the volumes of the gas decreases.
The formula for Boyle's Law is
In the formula...
P1 = The original pressure of the gas
V1 = The original volume of the gas
P2 = The final, or new, pressure of the gas
V2 = The final, or new, volume of the gas
History of Boyle's Law
- Also referred to as the Boyle-Mariotte Law due to the contribution of French physicist, Edme Mariotte.
- Created by Robert Boyle, an Irish physicist, chemist and alchemist.
- Robert Boyle worked along side Robert Hooke, creator of Hooke's Law (relation between force and elasticity).
- Robert Boyle proved his law by using the now-famous Mercury U-Tube experiment.
- Robert Boyle's study of gases and his gas law helped to develop the atomic theory.
- Robert Boyle published Boyle's Law in 1662.