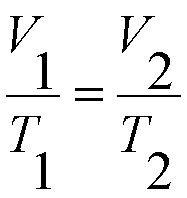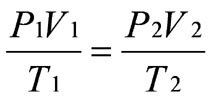Boyle's Law: This law states states, or explains, the inversely proportional relationship between a gas' volume and pressure at a constant temperature.
Charles' Law: This law states, or explains, the proportional relationship between a gas' volume and temperature at a constant pressure.
Combined Gas Law: This law states, or explains, that the volume of a gas is directly proportional to the temperature but inversely proportional to the pressure.
So how do they relate to each other?
The Combined Gas Law is a combination of both Boyle's Law and Charles' Law (as well as Gay Lussac's Law which is not covered in this website)
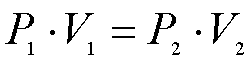
Boyle's Law
+
Charles' Law
=
Combined Gas Law