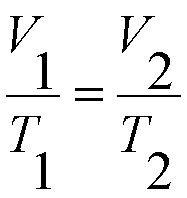For example, if you double the temperature from 100K to 200K, at a constant pressure, the volume will double as well.
This means the following conditions are required:
- The amount of gas is definite.
- The pressure (in mmHg, atm, kPa, etc) is constant.
- The gas is in a closed system.
According to Charles' Law
- As the volume increase, the temperature increases.
- As the temperature decreases, the volume decreases.
The formula for Charles' Law is
In the formula...
V1 = The original volume of the gas
T1 = The original temperature of the gas
V2 = The final, or new, volume of the gas
T2 = The final, or new, temperature of the gas
History of Charles' Law:
- Created by Jacques Charles, a French balloonist who flew the first hydrogen balloon in 1783.
- Charles experimented by filling five different balloons with the same volume of the five different gases and heated them each at the same time
- As he was doing his experiment, Charles saw that the balloons grew proportionally
- The final observation was not published until 1802 by Gay-Lussac
- Lussac named the law after the original scientist, Jacques Charles.